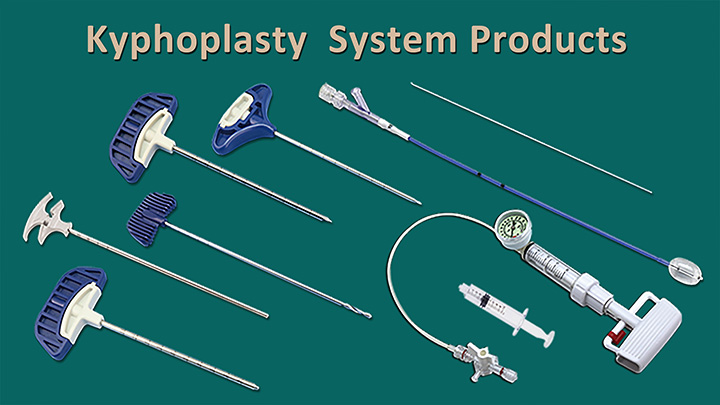
Landmark Success: Obtaining EU MDR CE Certificate
Jiangsu Changmei Medtech Co., Ltd.'s achievement in obtaining the EU MDR CE certificate for its Kyphoplasty System Products(kyphoplasty balloon catheter, kyphoplasty tool kit, and balloon inflator) represents a watershed moment in the company's journey toward global recognition and success.




