Changmei Medtech
Mark Chen
Oct
23
Dilation Balloon Catheter
Intended use: This devices is mainly used for dilating the digestive tract stricture and adjuvant therapy.
Features:
- Elastic soft tip design, which can smoothly enter into the target position with less tissue damage;
- The balloon is of extra transparent, which is convenient for observing during the dilation process;
- The balloon is using imported material with high pressure resistance, less deformation and safer dilation;
- Thermostatic shaping after multi-wing pleating contributes to excellent resilience and flexible retreat from working channel;
- Optimal design of the tube is smooth and of good elasticity, strong twisting resistance and easier passability;
- The radiopaque markers on the balloon can provide precise positioning under X-ray.
Intended use: This device is endoscopically used in digestive system or respiratory tract to guide other devices.
Features:
- Anti-twisting and high elastic NiTi core wire provides excellent torsional rigidity and can pass through complex anatomical structures
successfully; - Ultra-smooth PTFE coating is convenient for insertion, and bi-color helix design provides clear judgment of motion;
- Hydrophilic and radiopaque soft head design ensures smooth movement and accurate positioning under X-ray;
- Soft and round tip design reduces tissue damage of the patients;
- Patented soft tip connection technology provides multiple protection, thus it is impossible to break off;
- Various specifications are available according to clinical requirements, such as stiff guide wire, plastic coated guide wire, zebra guide wire, etc.
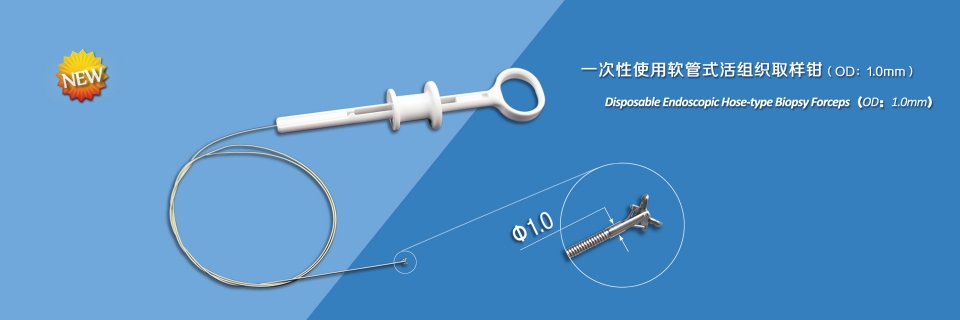
Intended use: This device is used endoscopically to obtain mucosal tissue biopsies. Sterile, for single-use.
Features:
- The jaws are made from medical stainless steel, and the head is carefully polished so that less damage is made to endoscope channel;
- Special processing of the cutting edge makes it sharp, consistent with each other, and sampling easily and reliably;
- Laser welding ensures high connection strength between components;
- Ultra smooth plastic layer of spring effectively reduces the damage to endoscope channel;
- Optimal design of handle makes the open or stop limit very clear and feels comfortable;
- Sterile package, disposable.
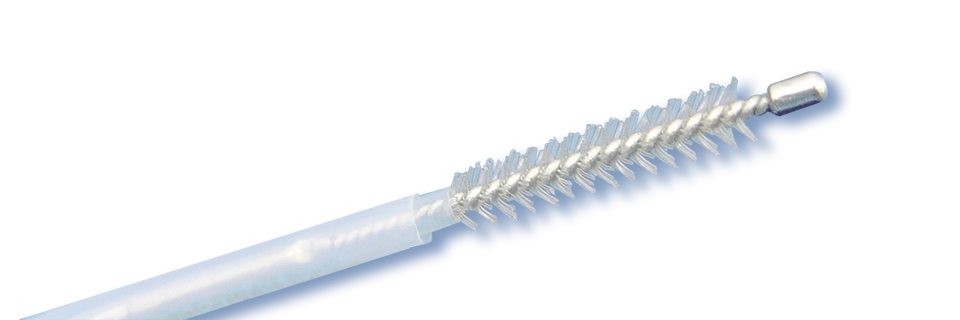
Intended use:The device is clinically used to brush cell sample.
Features:
- Unibody design of brush and pulling wire ensures that the brush will not fall off;
- Use imported bristles, which are neither too hard nor too soft, can brush cells easily and sufficiently;
- Straight shape brush head is smooth and round, effectively protect human tissues and endoscope channel;
- U-shape brush can rotate 360°to brush samples as much as possible, and can also be used for smear and cultivation;
- Sterile package, disposable.
Oct
23
Balloon Inflator - A type
Balloon Inflator (CE MDR Certificate and FDA Approval)
Intended use:
This device is mainly used for hydraulic pressure on balloons to achieve the function of balloon dilation.
Features:
- Optimal head fluid channel design makes it convenient for venting;
- O-type plunger design makes for low frictional resistance and can pressure easily;
- Screw fixed design of pressure gauge assures safety and reliability of the seal;
- The inflator is equipped with fluorescent gauge, which contributes to accuracy of reading;
- Ergonomic handle is comfortable and convenient to grasp;
- The inflator is of good leakproofness since it uses materials with high strength and safety;
Specifications:
| Model | Nominal Capacity(ml) | Maximum Pressure(atm) | Syring Features |
| BI-20A-10 | 20 | 30 | 10ml |