EUROSPINE 2025, here we come, waiting for you at booth #FG20
Welcome To The Eurospine 2025
Our booth number: 7.2b Budapest #FG20
October 22–24, 2025
Bella Center, Copenhagen, Denmark
Official exhibition website: https://www.eurospine.org/
Jiangsu Changmei Medtech Co., Ltd. specializes in the development, design and manufacturing of minimally invasive medical device products and related production equipment. It has a modern standard factory building of 6,800 square meters and a GMP standard 100,000-level clean workshop of 2,000 square meters. It has passed the South German TUV 13485 quality Management system certification, dozens of Class II and III domestic and foreign medical device registration certificates, and multiple patents and honors. The company focuses on the design, development and industrialization of overall solutions for medical balloon catheters and peripheral products, and is committed to becoming an international leader in this segment and providing high-quality products and services to domestic and foreign medical device companies and patients.

Welcome To The Eurospine 2025
Our booth number: 7.2b Budapest #FG20
October 22–24, 2025
Bella Center, Copenhagen, Denmark
Official exhibition website: https://www.eurospine.org/

Welcome To The North American Spine Society (NASS 2025)
Our booth number: #851
November 14-16, 2025
Colorado Convention Center Denver, CO, USA
Official exhibition website: https://www.spine.org/AM

Welcome To The German Congress For Orthopedics And Trauma Surgery(DKOU 2025)
Our booth number: #B38
CityCube Berlin, Germany October 28–31, 2025
Official exhibition website: https://dkou.org/en/

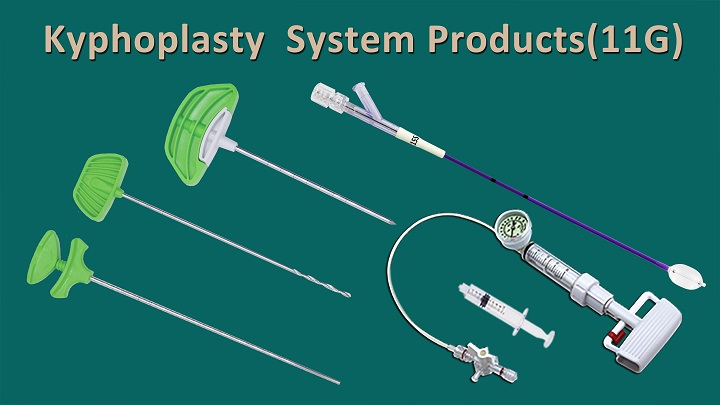
We are delighted to announce that CHANGMEI's kyphoplasty system products have successfully obtained FDA approval. This significant milestone includes our kyphoplasty balloon catheter, kyphoplasty tool kit, and balloon inflator.
This approval is a testament to our commitment to providing high-quality medical devices that meet stringent regulatory standards. It reflects our dedication to innovation and excellence in the field of orthopedic surgery.
We are excited about the opportunities this approval brings and look forward to offering our enhanced product line to healthcare professionals and their patients.
Thank you for your continued support and confidence in CHANGMEI.
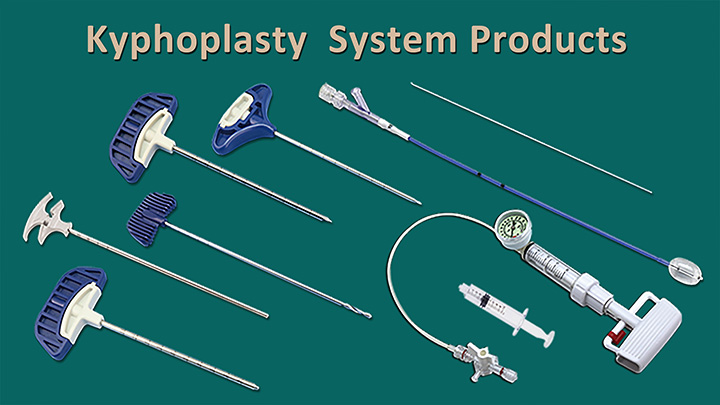
Jiangsu Changmei Medtech Co., Ltd.'s achievement in obtaining the EU MDR CE certificate for its Kyphoplasty System Products(kyphoplasty balloon catheter, kyphoplasty tool kit, and balloon inflator) represents a watershed moment in the company's journey toward global recognition and success.

Scan the QR code to download Changmei product brochure, Kyphoplasty System Products and Endoscopic Products Brochure.

The sun is just sailing in, and the dream is built together. On the morning of July 3, the groundbreaking ceremony of the West Taihu Plant of Jiangsu Changmei Medtech Co., Ltd. [WinMed Ultra-high Pressure Balloon R&D and Industrialization Project] was successfully held. Changmei medical management personnel, construction unit, design unit, project management company leaders participated in this event. Yuan Luxin, general manager of Jiangsu Changmei Medtech Co., Ltd., Fang Peiqi, chairman of Jiangsu Tianqi Construction Co., Ltd., Liu Kang, general manager of Jiangsu Yingte Engineering Consulting Design Management Co., Ltd., and Wang Junjie, general manager of Jiangsu Baoji Construction Project Management Co., Ltd., all came to the stage to deliver speeches. He expressed his congratulations and expressed his willingness to cooperate with each other, do a good job in detail, and welcome the perfect completion of Jiangsu WinMed Medical's ultra-high pressure balloon research and development and industrialization project in May 2023.
Prof. Dr. Tariq Sinan, consultant minimally invasive spine specialist in Kuwait has recently made a break through in treating an old patient with 2 fracture in lumbar verebrae due to RTA. One was shattered with broken wall. Patient was not fit for regular surgery (80 years old), he was suffering in an orthopedic hospital. His family came to Prof. Dr. Tariq who transferred the patient to hospital and on the same day he performed a revolusanry technique to treat the shattered fracture using CHANGMEI's Kyphoplasty Kit (Including balloon, tool kit and Inflator).

Nov. 26, 2016 was a happy day for celebrating becase the self-designed, developped and manufactured medical balloon catheter production line was delivered to a domestic well-known colleges successfully.

Jiangsu Changmei has launched two new products, which are 1.0mm biopsy forceps and 3-stage dilation balloon catheter. During Shanghai Medtec and Dusseldorf Medica expo, our new products were very popular within the customers from all over the world. They not only admire our technology strength and innovation capability, but also make an order on the spot. The great feedback from the market earns more confidence and determination for the following new product research and development.

The self-developed Balloon Forming Machine (Changmei Fourth Generation ) has been delivered to American client by DHL on 8th June.