Particle Filtering Half Mask
Product Introduction
Name: Particle filtering half mask FFP2 NR
Model: DFM-01
Following Standard: EN 149:2001+A1:2009
Following Regulation: (EU)2016/425
Intended use:
Particle filtering half mask is minimum requirement as respiratory protective devices
to protect against particles except for escape purpose.
Manufacturer: Jiangsu Changmei Medtech Co.,Ltd.
How to use
Use Instruction
1.Failure to follow all instructions and limitations could seriously reduce the effectiveness of this particle filtering half maskand could lead to illness, injury or death.
2.A properly selected particle filtering half mask is essential, before occupational use, the wearer must be trained by the employer in the correct use of the particle filtering half mask in accordance with applicable safety and health standards.
3.This particle filtering half mask does not supply the Use only in adequately ventilated is as containing sufficient oxygen to support life.
4.Discard the particle filtering half mask and replace with a new one ifexcessive clogging of the particle filtering half mask cause breathing difficulty or the particle filtering half mask becomes damaged.
5.Leave the contaminated area if dizziness, irritation or other distress occurs.
Use Limitation
1. Do not use the particle filtering half mask or enter or stay in a contaminated area under the following circumstance:
a)Atmosphere contains less than 19.5% oxygen.
b)If you smell or taste contaminant.
c)For protection against gases or vapors.
d)Contaminants or their concentrations are unknown or immediately dangerous to life or health.
e)For sandblasting, paint-spray operations and asbestos.
f)In underwater, fire and explosive atmospheres.
2.Do not modify or misuse the mask.
3.Do not use theparticle filtering half mask with facial hair or any other conditions that may prevent a good face-seal, the requirements for leakage will not be achieved.
4.Particle filtering half mask need to be inspected prior to each use to assure there are no holes in the breathing zone other than punctures around and staples and no damaged has occurred. Enlarged holes resulting from ripped or torn filter material around staple punctures are considered damage.
5.Thisparticle filtering half mask helps protect against certain particulate contaminants but does not eliminate exposure to the risk of contracting disease or infection. Misuse may result in sickness or death.
6.This particle filtering half mask marked “NR”, shall not be used for more than one shift.
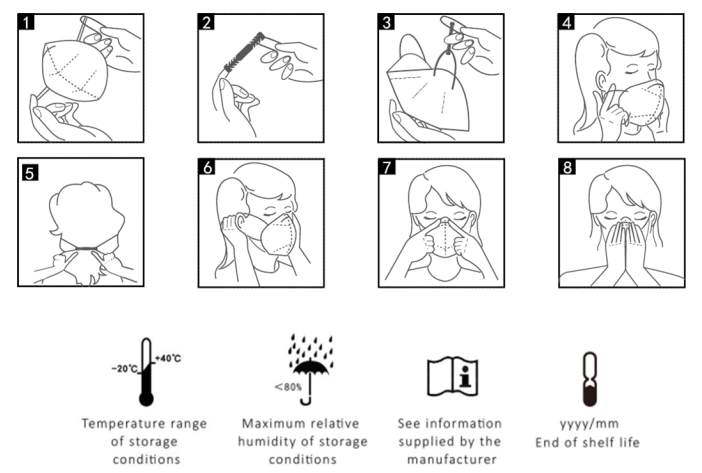
Fitting Instruction
1.Open the particle filtering half mask, face to the inside of the mask, and hold the mask on each hand so that the nose clip is at the top.
2.Take out the retaining clip from the package and fasten one end of the retaining clip to one side of the mask.
3. Hold the particle filtering half mask in position over the nose and mouth, and fasten the other end of the retaining clip to the other side of the mask.
4. Adjust to a comfortable position and make the mask fit the face.
5. Bend the nose clip to make a tight seal around the nose.
6.Fitcheck
a)To test the fit of the particle filtering half mask, cup both hands over the particle filtering half mask and inhale sharply. If air flow is felt in the nose area, re-adjust/tighten the nose clip.
b)If flows is felt around the edges of the particle filtering half mask, re-position the mask harness to achieve a better fit.
7.Change the particle filtering half mask immediately if breathing becomes difficult or particle filtering half mask becomes damaged or distorted.
8.Change the particle filtering half mask if a proper face seal cannot be achieved.
9.Careful observances of these instructions is an important step in safe particle filtering half mask use.
Notified Body: CCQS Certification Services Limited
NB: 2834
Address: Block 1 Blanchardstown Corporate Park, Ballycoolin Road, Blanchardstown, Dublin 15, D15 AKK1, Ireland
Manufacturer:Jiangsu Changmei Medtech Co., Ltd.
Address: No.27, Xinke West Road, Luoyang, 213104 Changzhou City, Jiangsu Province, P. R. China
Additional Info
- Test for FFP2 NR mask Test report for FFP2 NR mask download(PDF)
- DOC of FFP2 NR face mask Doc of FFP2 NR mask download(PDF)
Intended use:
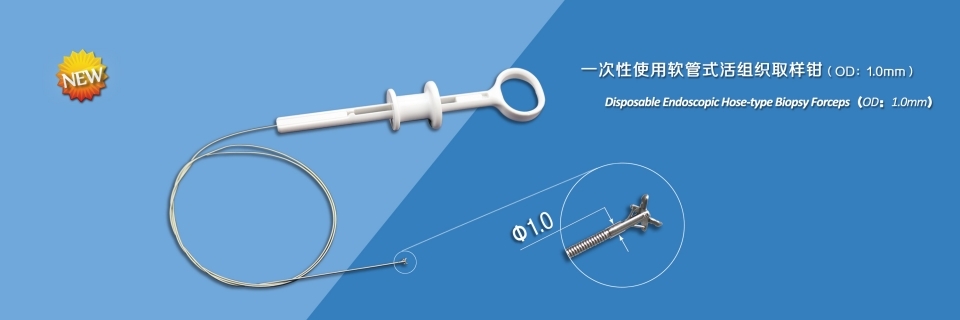
This device is used endoscopically to obtain biopsy samples from digestive tract and respiratory tract.
Features:
- The jaws are made from medical stainless steel, and the head is carefully polished so that less damage is made to endoscope channel;
- Special processing of the cutting edge makes it sharp, consistent with each other, and sampling easily and reliably;
- Laser welding ensures high connection strength between components;
- Ultra smooth plastic coating of spring effectively reduces the damage to endoscope channel;
- Optimal design of handle makes the open or stop limit very clear and feels comfortable;
- Sterile package, disposable.
Sepeification:
| Model | Jaws Diameter | Channel I.D. | Working Length | Cups Shape | Coating | Needle |
| FB-12E-B1 |
1.0mm |
≥1.2mm |
1200mm |
Alligitor Cups |
No |
No |
| FB-12N-B1 | 1800mm | |||||
| FB-12U-B1 | 2300mm |
E-Brochure of SpyGlass™ DS and SpyBite™ Biopsy Forceps From Boston Scientific
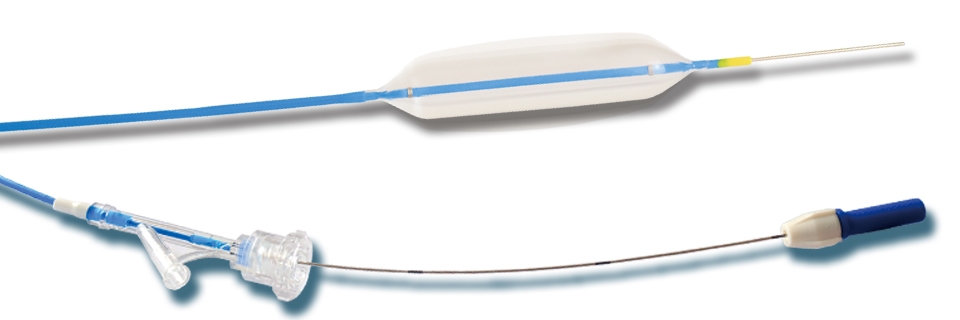
Intended use:
It is suitable for adults and adolescents in the dilation operation of digestive tract stricture under endoscopes.
Features:
- The balloon can be gradually increased at three distinct diameters under specific pressure, providing much more choice for physicians.
- Elastic soft tip design, which can smoothly enter into the target position with less damage.
- Rapid drainage design helps to reduce surgery time.
- Use imported material with high pressure resistance and safer dilation.
- Thermostatic shaping after multi-wing pleating contributes to excellent resilience and flexible retreat from working channel.
- Optimal design of the tube makes it smooth and of good elasticity, strong twisting resistance and easier passability.
- The radiopaque markers on the both ends of the balloon can provide precise positioning under X-ray.
- Pre-input 0.035” guide wire with soft tip and mark, which is easier to estimate the length of the inside guide wire.
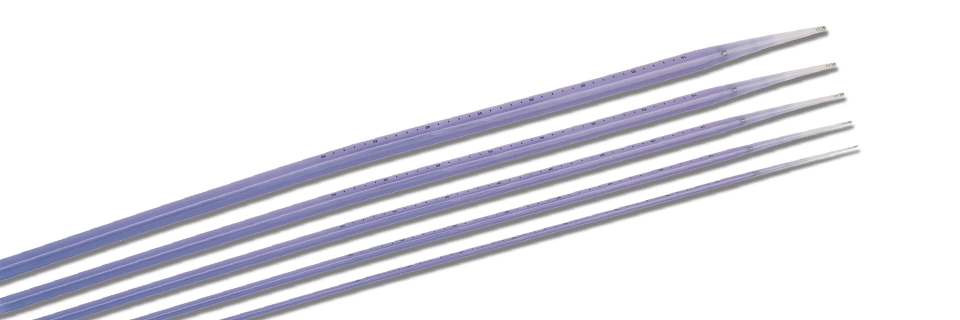
Bougie Dilator Sets
Intended use:
It is used for the expansion and treatment of esophagus and cardia.
Features:
- Made by medical polymer materials with proper softness.
- Anti-shedding design, more safety and reliable.
- The front cone angle is manufactured by mechanized processing. With smooth transition, it can be inserted into the narrow parts successfully.
- Two radiopaque marks on both ends make it easy to observe under X-ray.
- Print scales on both sides, allowing the physicians to identify the insertion length.
Intended use:
This device is mainly used to remove bile duct stones or foreign bodies in upper and lower digestive tract.
Features:
- With injection port in handle, it is convenient to inject contrast medium smoothly.
- Excellent delivery performance makes it easier to reach the target.
- Push-pull handle together with rotatable design makes stone grabbing more easy and effective.
- Diamond shape basket is easily to grab and release, especially for repeat use.
- Memory alloy design of basket ensures that it can remain original shape after sophisticated stone removal.
- Three different types of basket are available to meet clinical requirements, including diamond, spiral and oval shape.
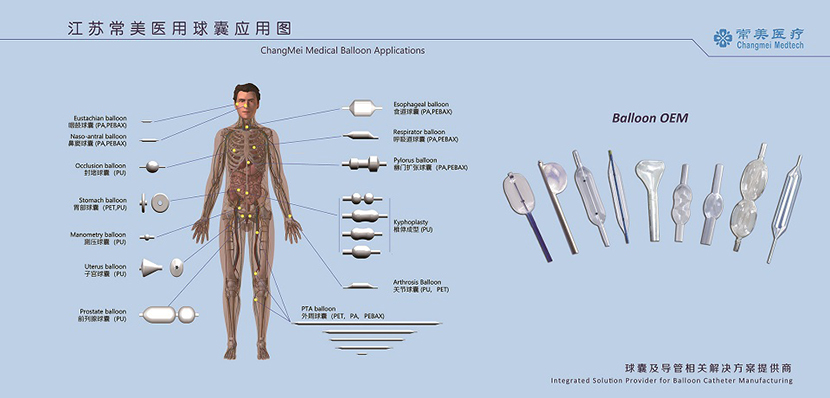
OEM Medical Balloon
We specializes in the development and design of medical devices, with a particular focus on medical balloon research and production. We can provide integrated solutions of balloon and catheter related products as well as full-set production equipment according to customer requirements.
Material: PA, PEBAX, PU
Brand: Lubrizol, Arekma, Evonik
Production Environment: 100 000 Class Clean Room
Main Equipments: 2 Balloon Tube Extrusion line, 5 Balloon Neck Forming Machine, 12 Balloon Forming Machine, other equipments such as Balloon Welding Machine, Rediopaque Swaging Machine, Balloon Pleating Machine, Balloon High Pressure Seal Tester, Tip Forming Machine


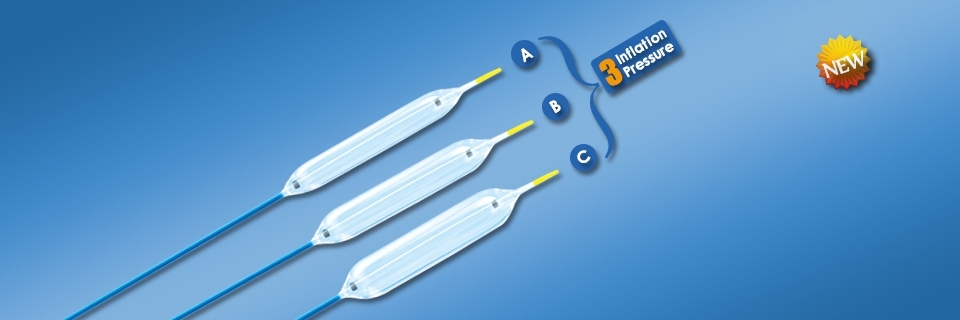
Intended use:This device is mainly used for adults and adolescents in the dilation operation of digestive tract stricture under endoscopes.
Features:
- 3-stage Dilation Balloon Catheter has three distinct diameters at three separate pressures during in vivo dilation, thus can achieve three kinds of conventional non compliant balloon dilation effect;
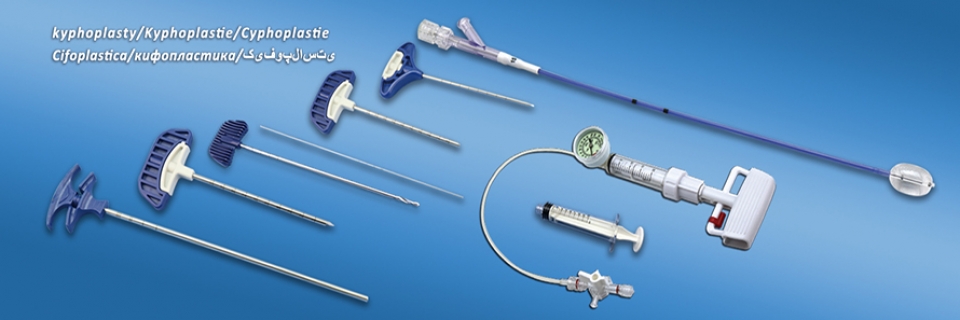
Kyphoplasty System Products
Components of Kyphoplasty System Products:
- Kyphoplasty Balloon Catheter: The device is mainly used in PKP operation to dilate vertebral body and form a cavity, which is for injecting bone cement to recover and stabilize vertebral body.
- Kyphoplasty Tool Kit: The device is mainly used for bone percutaneous intervention and establishing working channel by medical institutions.
- Balloon Inflator: The device is mainly used for hydraulic pressure on balloons to achieve the functure of balloon dilation.
Features:
- Minimally invasive surgery can reduce tissue injury and blood loss;
- The operation is simple, fast, and the operation time is greatly shortened;
- Local anesthesia can be adopted to reduce the risk of surgery and postoperative complications;
- The product can immediately relieve postoperative pain, effectively restore vertebral body height and correct spine kyphosis.
Kyphoplasty System Products List (8G)
| No. | Name | Model | Qty. |
| 1 | Kyphoplasty Balloon Catheter | KB0210/KB0115/KB0120 | 1 |
| 2 | Balloon Inflator | BI-20A-10 | 1 |
| 3 | Kyphoplasty Tool Kit | KT-00-01/KT-00-02/KT-00-03/KT-00-04 | 1 |
Kyphoplasty Tool Kit List (8G)
| No. | Name | Model | KT-00-01 | KT-00-02 | KT-00-03 | KT-00-04 |
| 1 | Puncture device | KT-01-01 | 2 | 2 | 1 | 1 |
| 2 | Puncture & Expandor device | KT-02-01 | 1 | / | 1 | / |
| 3 | Expandor device | KT-03-01 | 2 | 2 | 1 | 1 |
| 4 | Bone drill | KT-05-01 | 1 | 1 | 1 | 1 |
| 5 | Guide wire | KT-06-01 | 2 | 3 | 1 | 1 |
| 6 | Bone cement filling device | KT-04-01 | 6 | 6 | 3 | 3 |
Kyphoplasty System Products List (11G)
| No. | Name | Model | Qty. |
| 1 | Kyphoplasty Balloon Catheter | KB0210S/KB0115S/KB0120S | 1 |
| 2 | Balloon Inflator | BI-20A-10 | 1 |
| 3 | Kyphoplasty Tool Kit | KT-00-05/KT-00-06 | 1 |
Kyphoplasty Tool Kit List (11G)
| No. | Name | Model | KT-00-05 | KT-00-06 |
| 1 | Puncture & Expandor device | KT-07-01 | 2 | 1 |
| 2 | Bone cement filling device | KT-08-01 | 6 | 1 |
| 3 | Bone drill | KT-09-01 | 1 | 1 |
Intended use:The device is clinically used to brush cell sample.
Features:
- Unibody design of brush and pulling wire ensures that the brush will not fall off;
- Use imported bristles, which are neither too hard nor too soft, can brush cells easily and sufficiently;
- Straight shape brush head is smooth and round, effectively protect human tissues and endoscope channel;
- U-shape brush can rotate 360°to brush samples as much as possible, and can also be used for smear and cultivation;
- Sterile package, disposable.
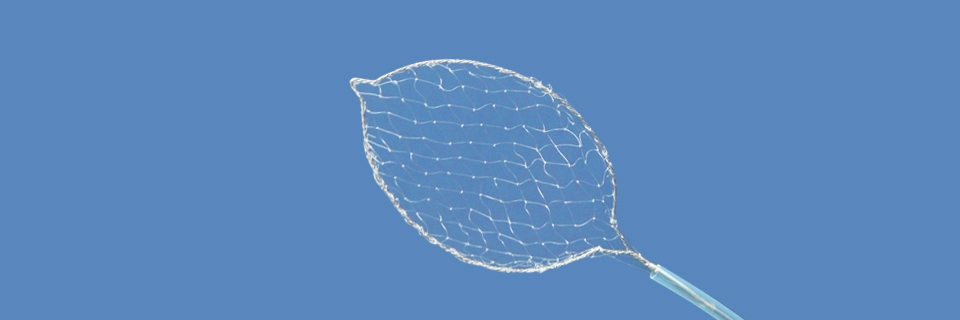
Intended use: This device is combined use with endoscope to extract and remove foreign body in digestive tract.
Features:
- Net design can reduce the risk of foreign body loss, and can be used to take out slithery and complicated foreign bodies;
- Using imported superfine silk net with good elasticity, high strength and excellent passability;
- 360 °rotating design brings convenience for clinical operation;
- Sterile package, disposable.